MedTech compliance made easy
With Kickfile, you get access to the systems you need for putting a medical device on the market – in a compliant and predictable way.
- Instant
- Accurate
- Flexible

What is Kickfile?
A document system supporting compliance for medical devices, IVD products, cosmetics & pharma. All processes, templates and forms needed for market access are aligned and updated according to the latest regulations and standards. In other words, everything you need for a more predictable way to market.
Why compliance?
In order to reach the market – compliance with laws and regulations is a must for medtech and pharmaceutical products. Regulatory bodies require traceable processes and documentation to ensure quality, performance, efficacy, and safety. Kickfile provides just the right documents and processes needed to get your product on the market and support you during an audit. Instant, accurate, and flexible.
How it works
Get the tools for a smooth and accurate compliance process.
1. Start by specifying product category and areas of interest
2. Choose access type
3. Request a quote
4. Download all files required
5. Ready to start
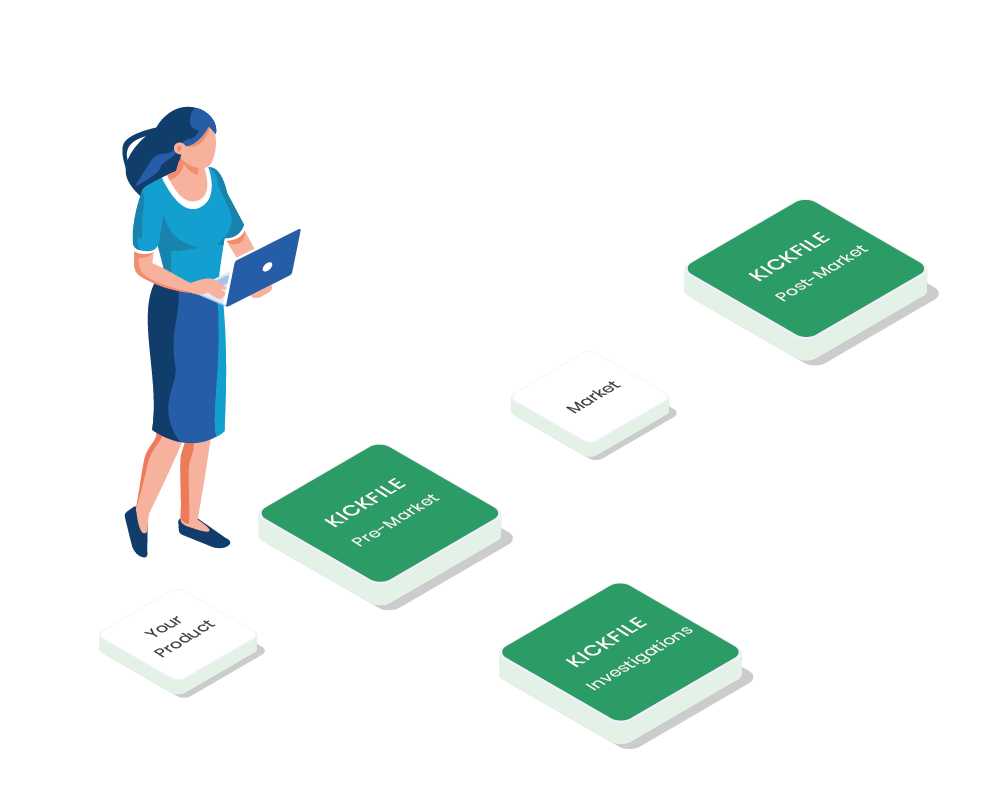
Workflow example
Kickfile offer support with e.g.
Clinical Evaluation
Whether you are developing a new medical device, or need to update an existing clinical evaluation, Kickfile’s system provides you with the tools you need to ensure compliance with MEDDEV 2.7/1 Rev4 and MDR 2017/745.
Clinical evaluation ensures the safety and performance of a medical device. It is an ongoing process to collect, appraise and analyze clinical data throughout the life cycle of a medical device.
Whether you are developing a new medical device, or need to update your clinical evaluation, Kickfile’s system provides you with the tools you need to ensure compliance with MEDDEV 2.7/1 Rev4 and MDR 2017/745.
Example of material:
- Clinical Evaluation Procedure
- Clinical Evaluation Plan Template
- Clinical Evaluation Report Template
- Clinical Development Plan Template
- Information Search Protocol Template
- Information Search Report Template
- State-of-the-Art Report Template
- Summary of Safety and Clinical Performance Template
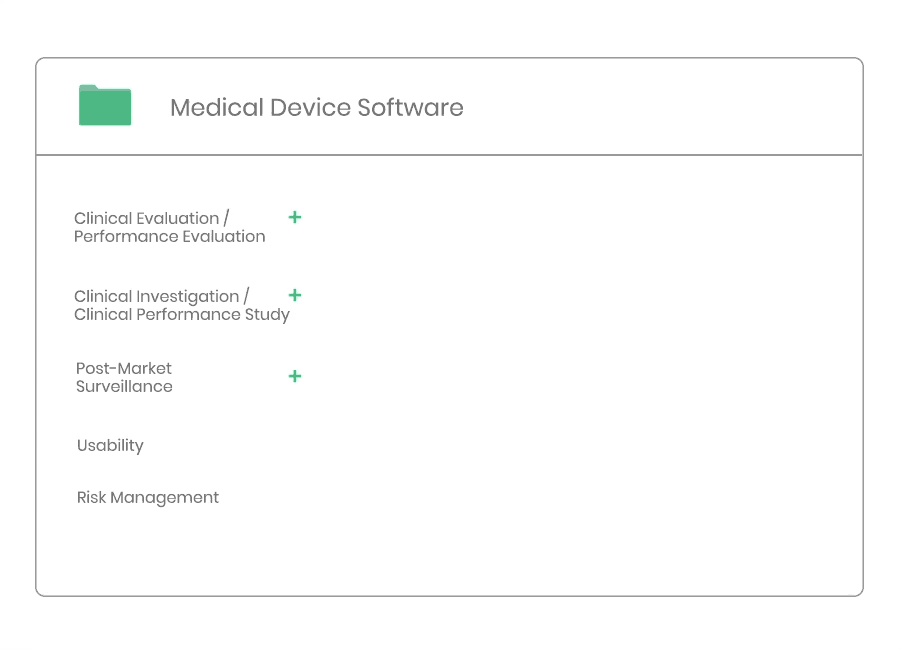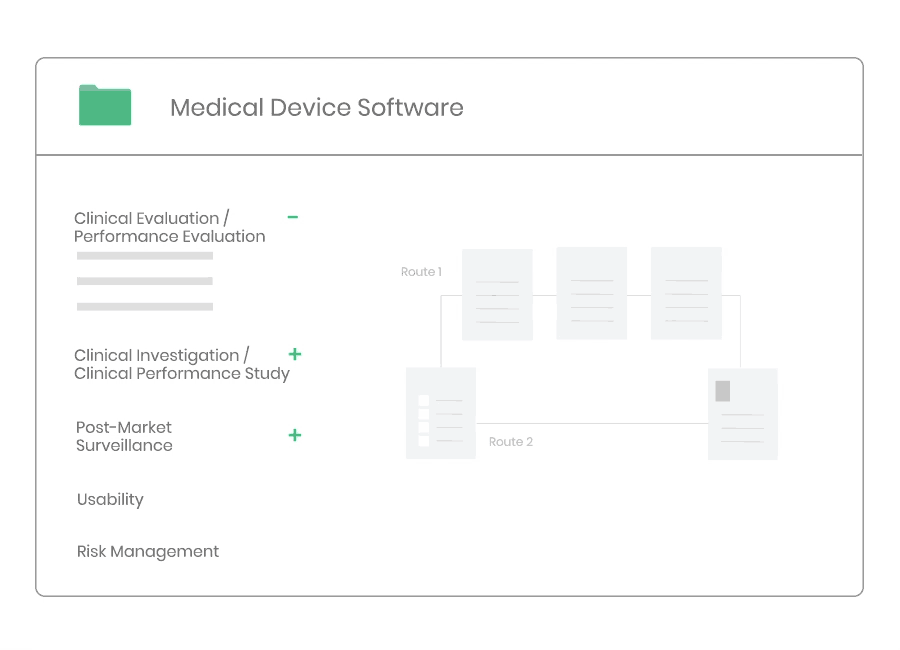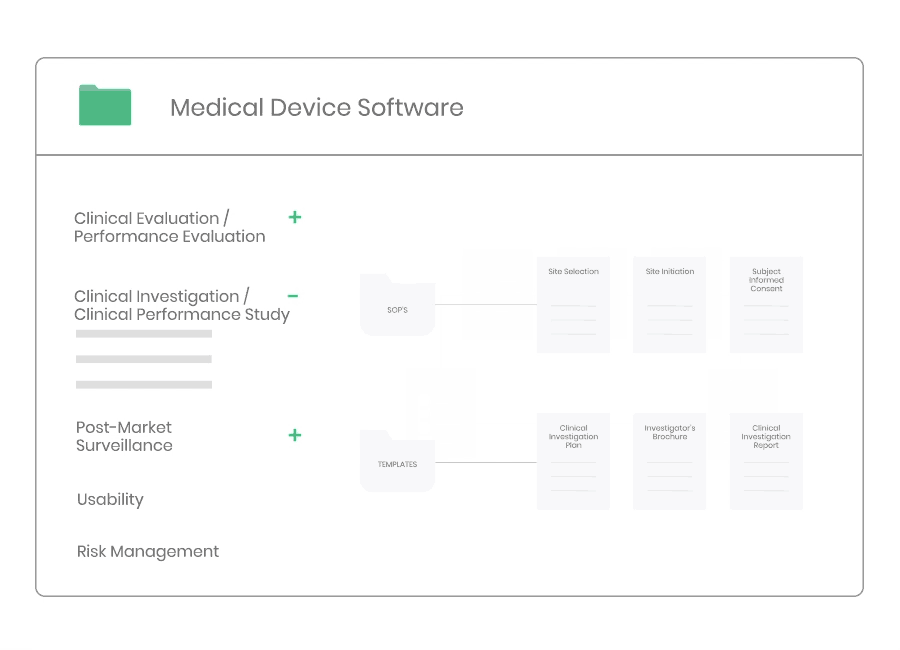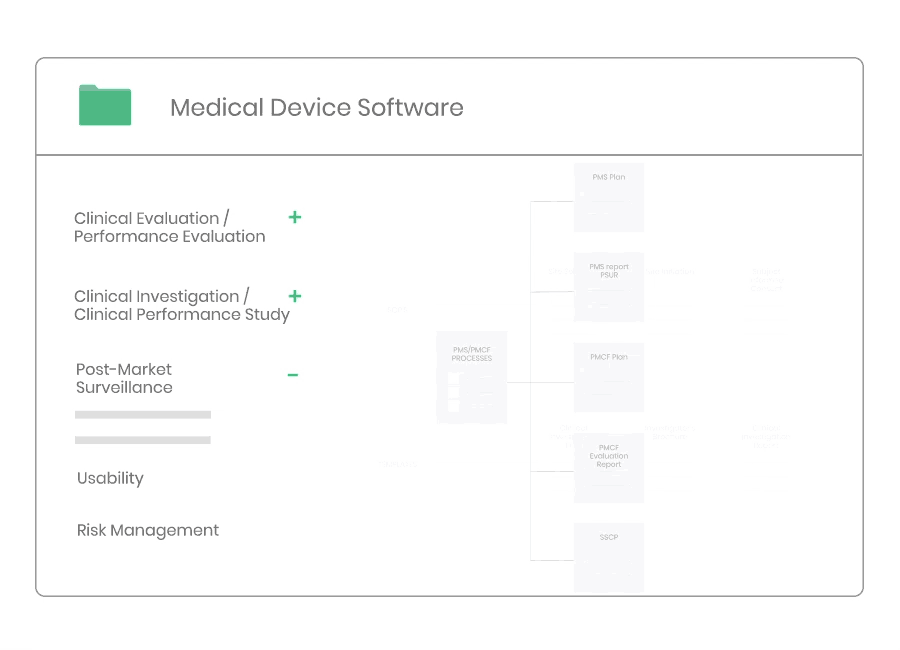
Clinical Investigation
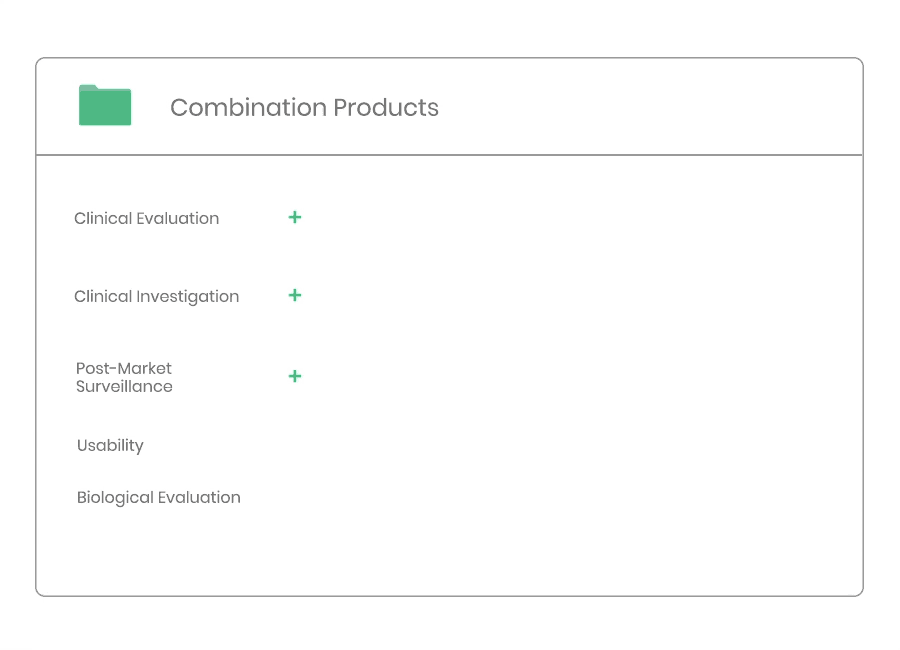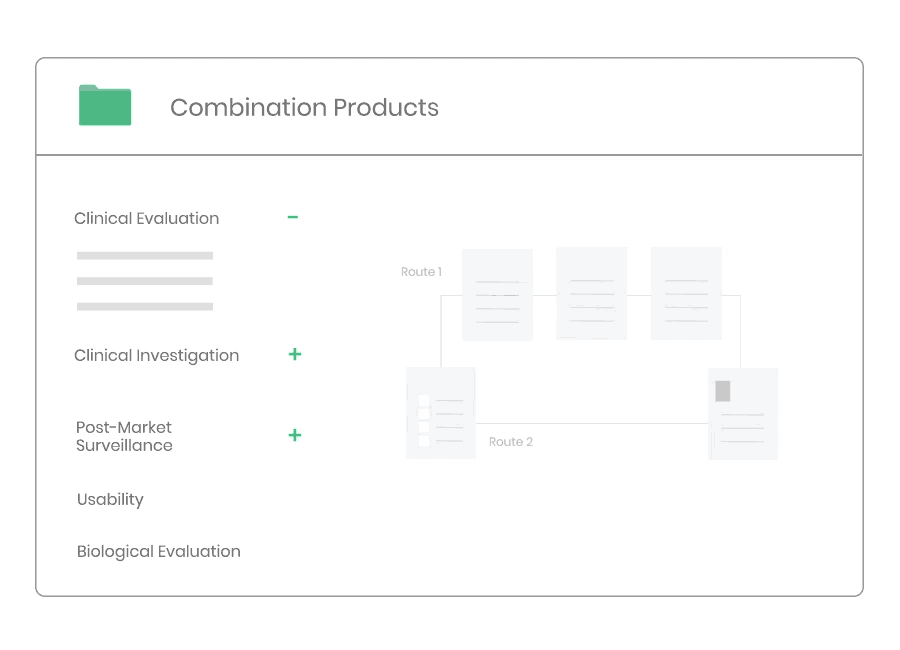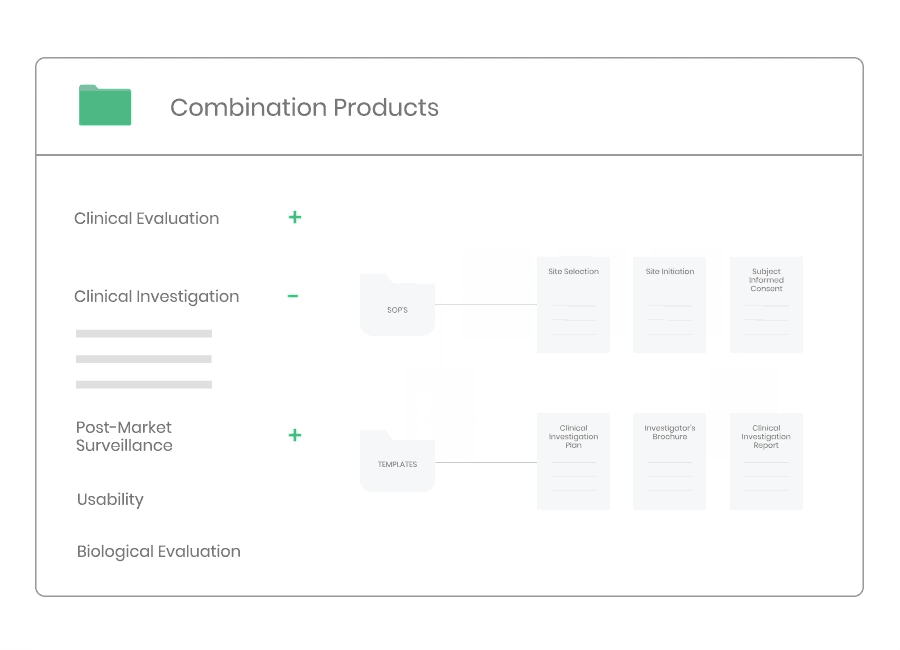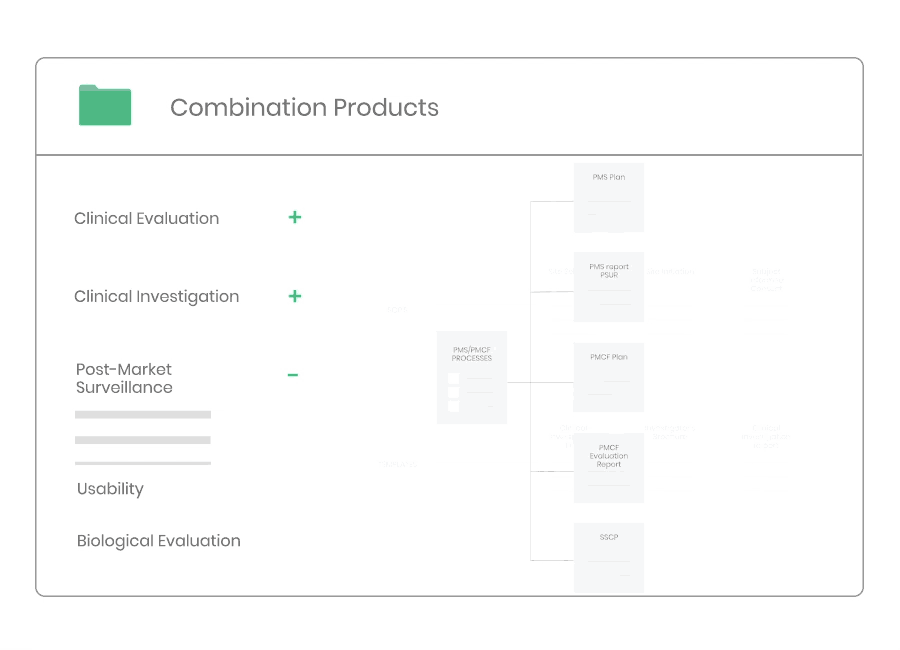
According to ISO 14155 and MDR 2017/745 the Sponsor must implement and maintain written quality procedures to ensure that the clinical investigation is designed, conducted and reported in a compliant manner.
Through Kickfile you access validated systems for all types of clinical investigations, e.g. company sponsored as well as investigator initiated investigations.
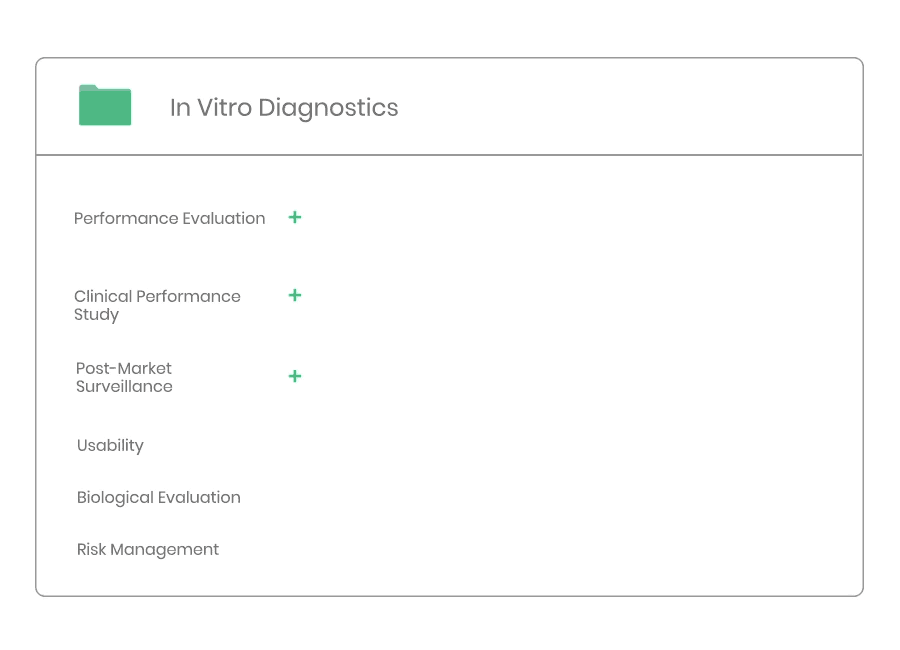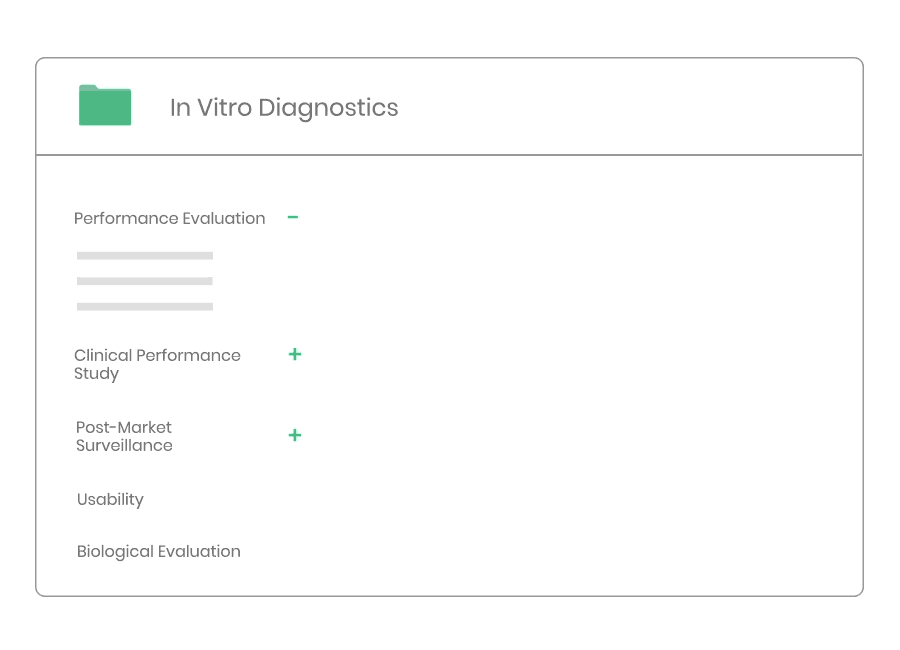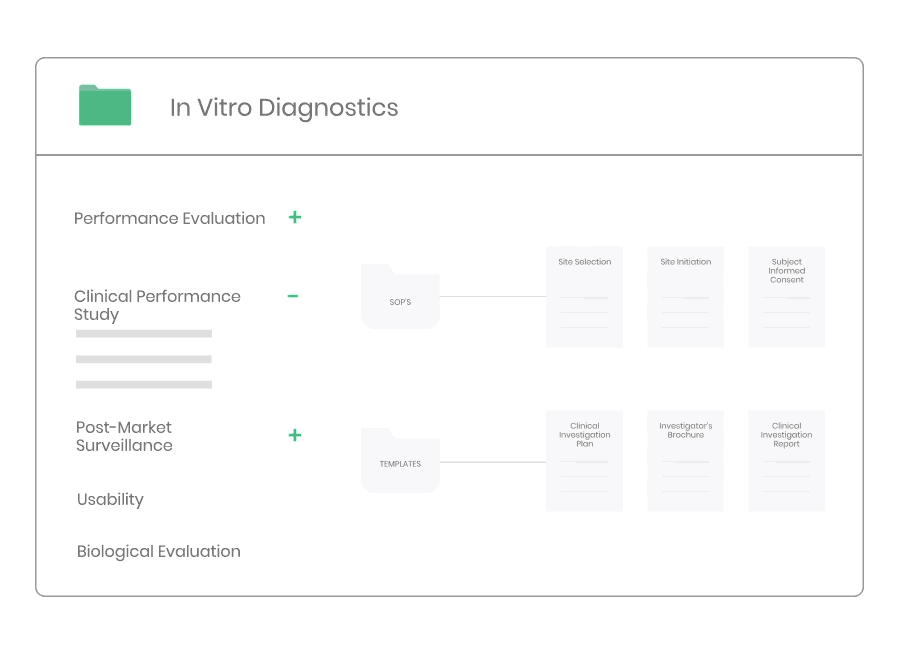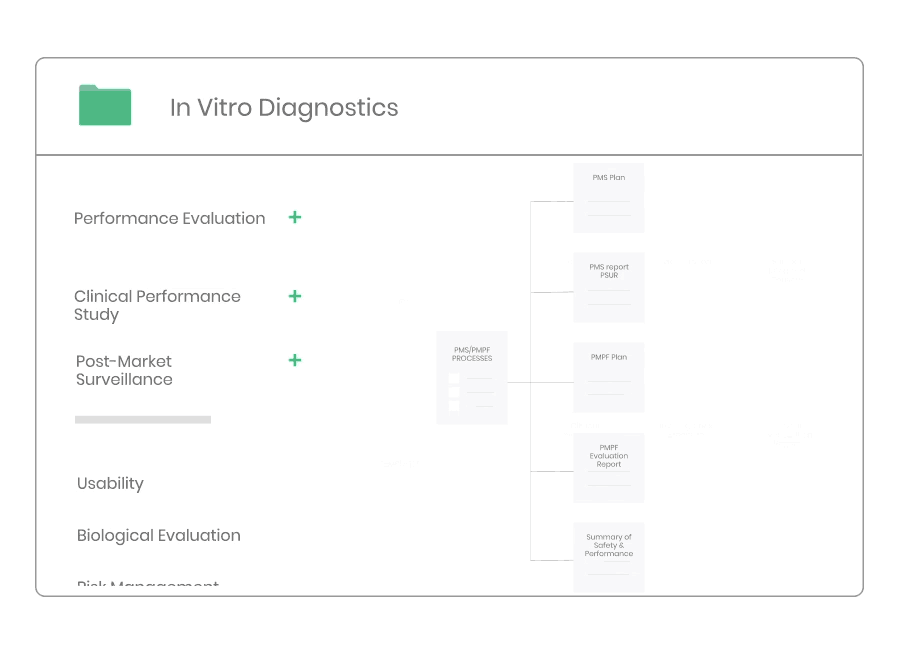
Example of material:
- 17 Procedures, incl. Site Selection, Informed Consent, Monitoring, and Safety Reporting.
- 34 Templates, incl. Clinical Investigation Plan, Investigator’s Brochure, Monitoring Plan, Clinical Investigation Report, Study Master File Index, and Monitoring Visit Report.
Post-market Surveillance
Regardless of medical device type or class you will need to have a dedicated PMS system to collect and review experiences gained from the device once placed on the market.
Kickfile’s systems can be used for creating PMS and PMCF plans, reports and other necessary documentation. The systems are always up to date with the latest regulations and guidelines and can be customized for your product and company.
Example of material:
- Post-Market Surveillance Procedure
- Post-Market Clinical Follow-up Procedure
- PMS Plan Template
- PMCF Plan Template
- PMS Report Template
- PSUR Template
- PMCF Evaluation Report Template
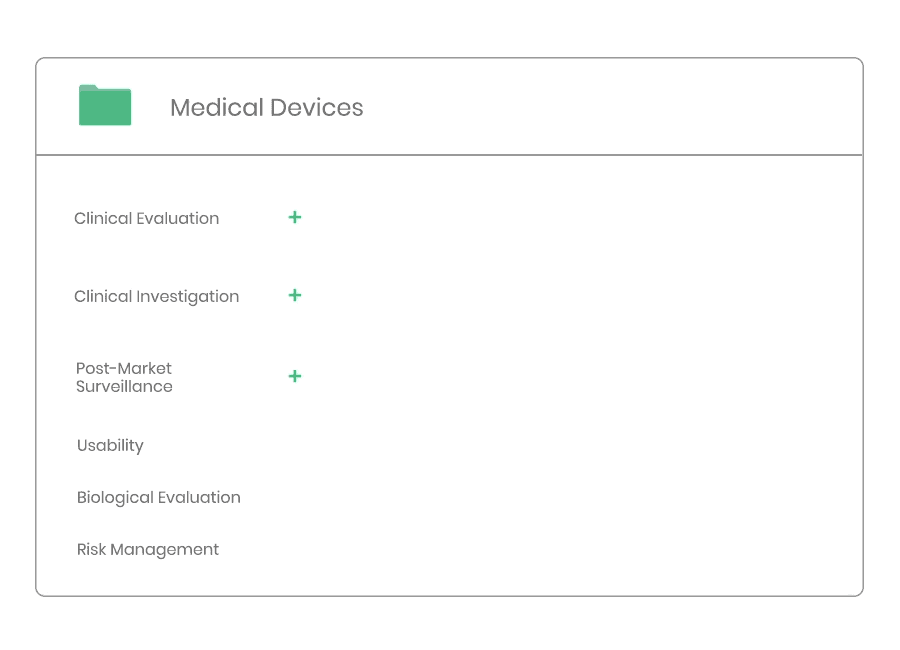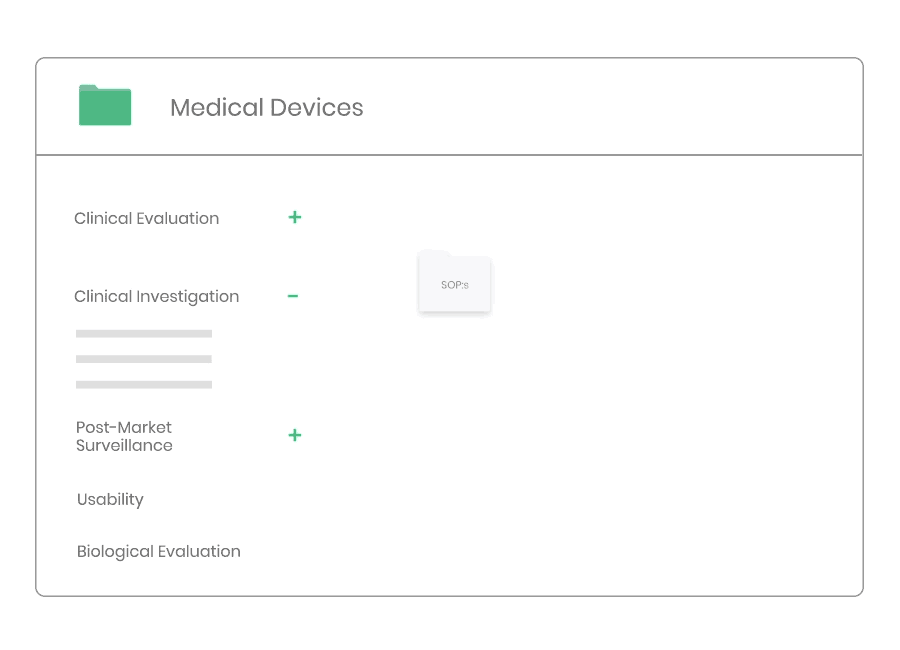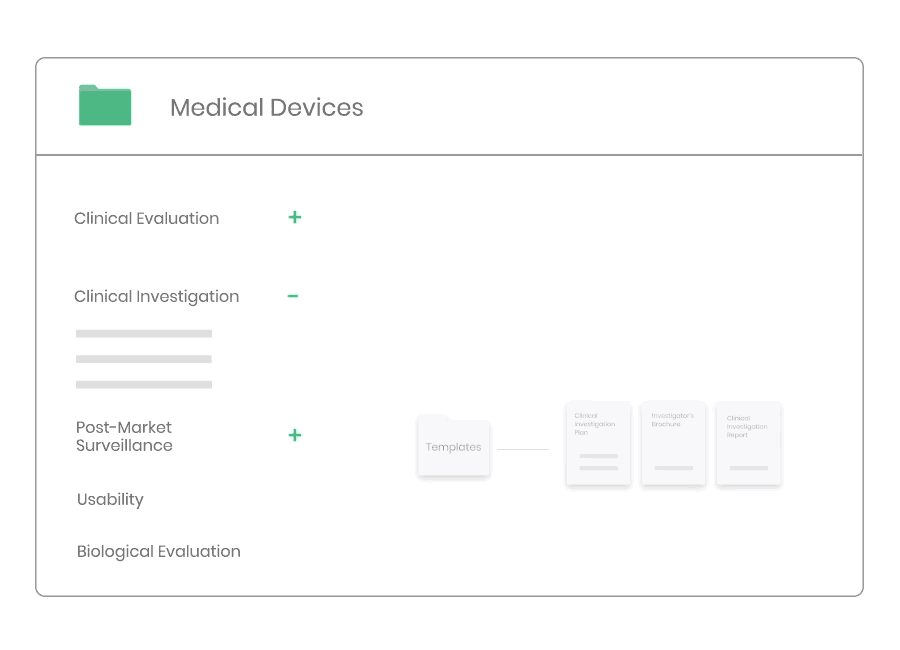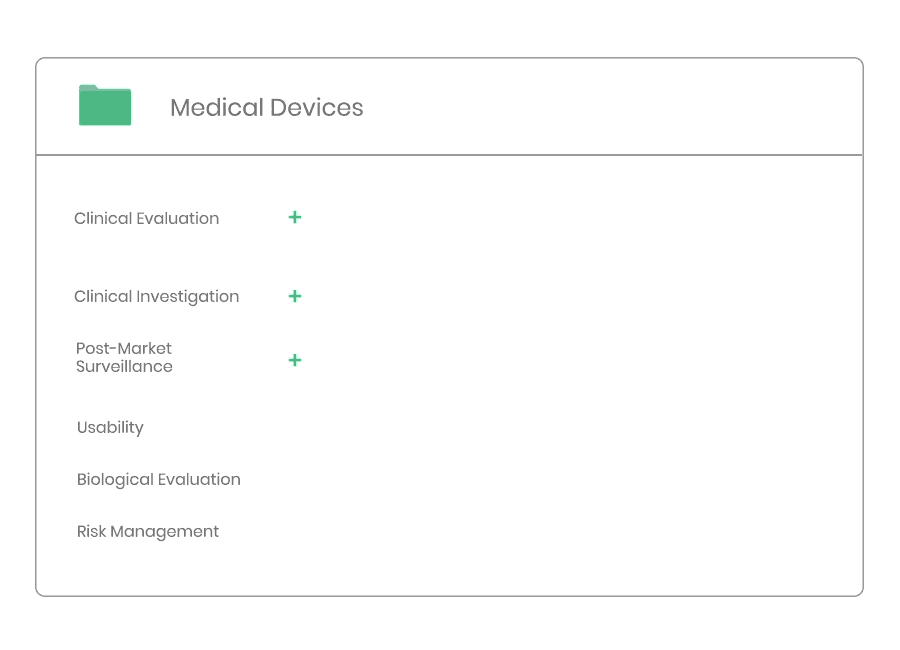
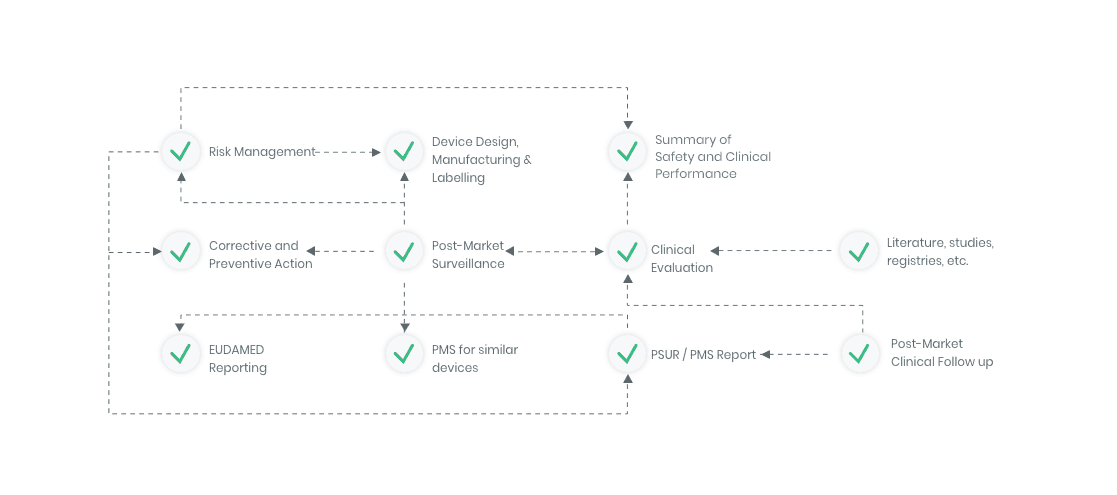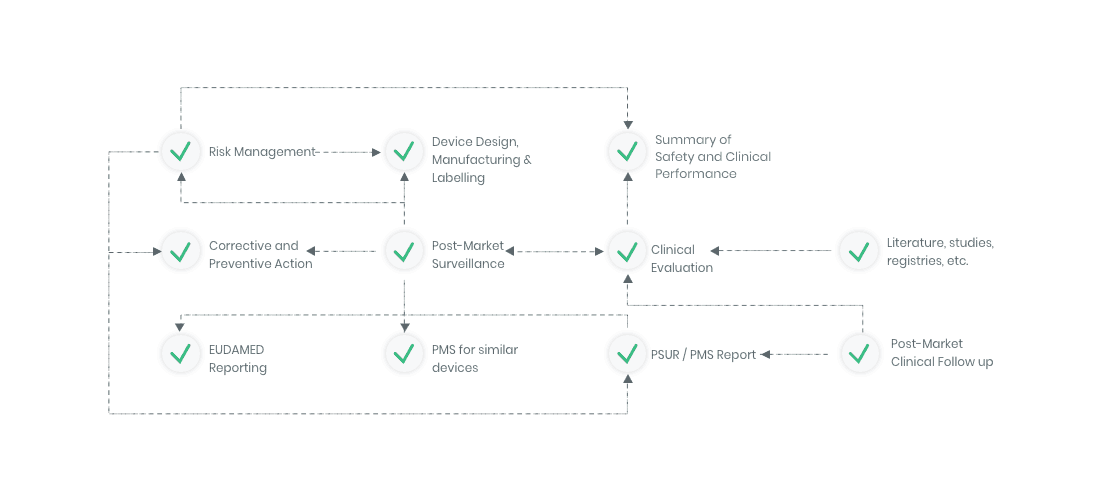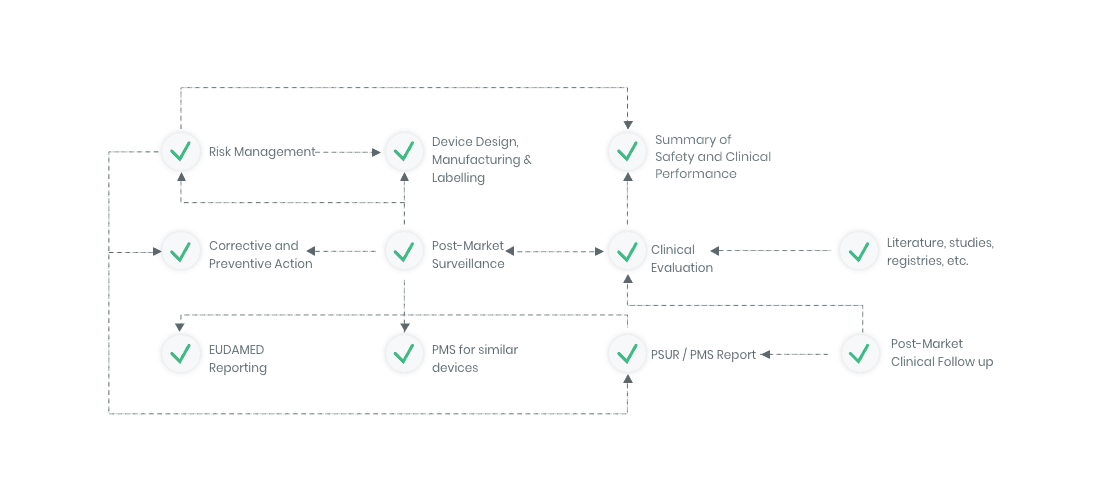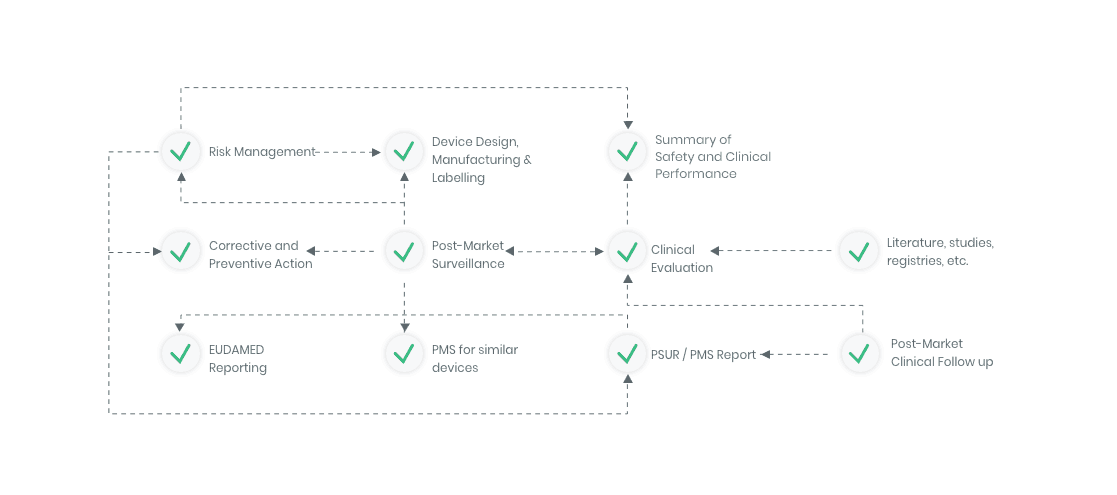
The compliance ecosystem
The Kickfile systems cover every step of the complex compliance ecosystem. You get support and guidance to the right strategies, structures and documents – making your process faster and more accurate.
“We established Kickfile to make it easy to become and stay compliant.”
Sofia Spjuth
CEO Kickfile AB
Why Kickfile?
With Kickfile you always have up-to-date documents and processes for a faster and more predictable market access.

Instant Access
With our tools you get immediate access to everything you need.

Accurate and proved
Our systems have been audited by external auditors and used operationally for several years.
Flexible
Only pay for what you need when you need it.

Unmatched expertise
Our experts educate notified bodies and take part in the working groups writing international standard for medical devices and IVD products.

Professional support
Through our network you gain access to some of the most experienced MedTech experts available.
Up to date
Through our systems you get access to the latest and most accurate documents.
News & Whitepapers
Partners for full service
We are your full-service medical device partners. Together we offer unique expertise regarding Quality Assurance, Regulatory Affairs, Clinical Affairs, Biological Evaluations & Toxicology.







![Clarvin-logo-Blå-NY[16]](https://www.kickfile.se/wp-content/uploads/2022/02/Clarvin-logo-Bla-NY16.png)